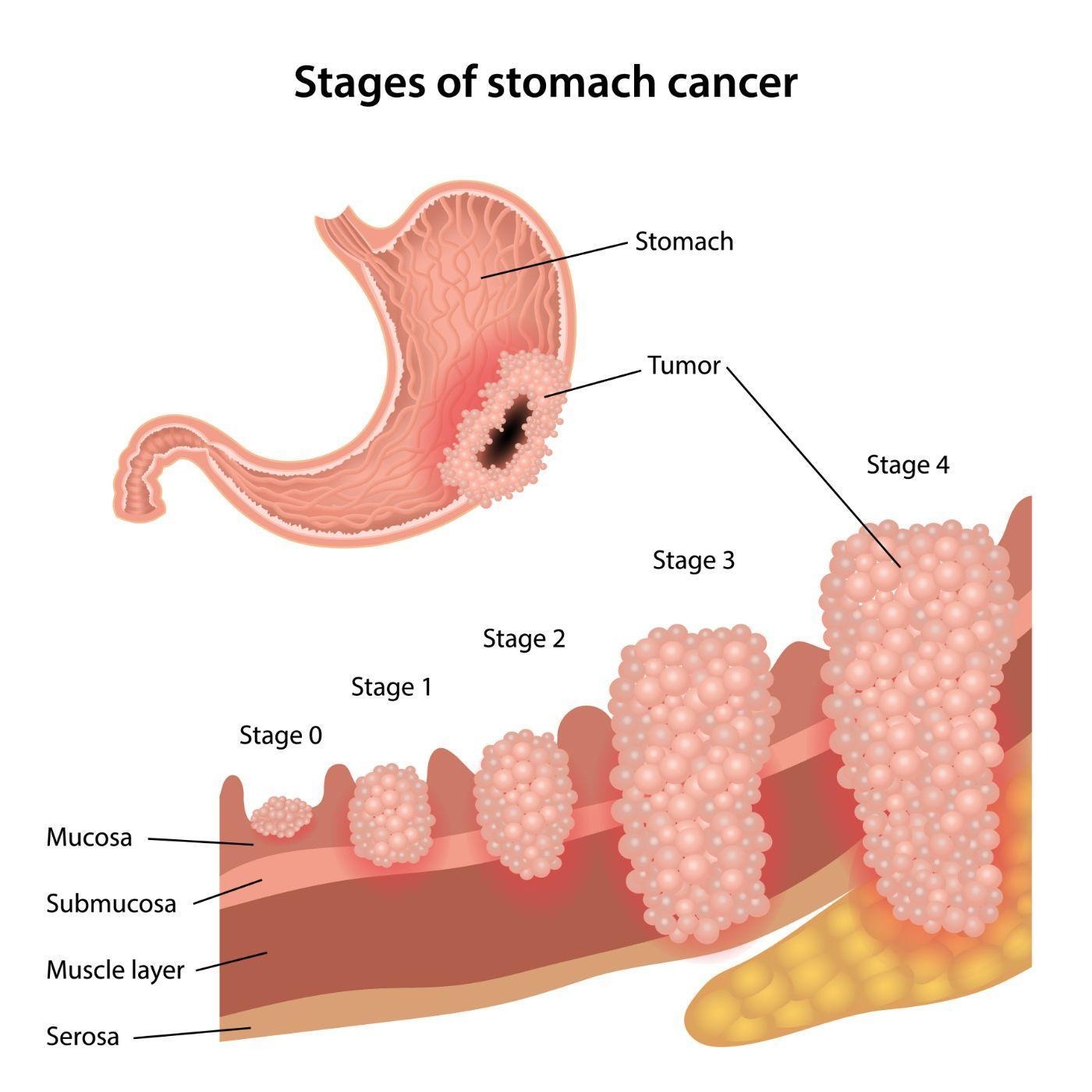
Background
- Gastric cancer is a malignancy arising from the stomach
- Gastric adenocarcinoma is the most common type of gastric cancer (90%)
- This condition can be divided into intestinal and diffuse gastric cancer types
- Intestinal gastric tumors are bulky and have glandular structures (most commonly found on the lesser curvature of the stomach)
- Diffuse gastric tumors are infiltration tumors and are composed of signet ring cells (stiffening of the gastric wall; also called linitis plastica is also found)
Version 2
Definition: Gastric cancer is a malignant neoplasm arising from the epithelial cells of the stomach lining.
Epidemiology:
- ★ Most common type: Gastric adenocarcinoma (90-95% of all gastric cancers)
- Incidence: ~1 million new cases annually worldwide
- ★ Most common location: Eastern Asia (Japan, Korea, China) and Eastern Europe
- ★ Most common age: 60-70 years (median age at diagnosis: 68 years)
- ★ Gender predilection: Male:Female ratio = 2:1
- ★ Most common site in stomach: Lesser curvature (40%), followed by cardia (35%)
Pathophysiology:
- Chronic inflammation → intestinal metaplasia → dysplasia → carcinoma (Correa cascade)
- Two main histologic types per Lauren classification:
- Intestinal type (54%): Well-differentiated, glandular structures, better prognosis
- Diffuse type (32%): Poorly differentiated, signet ring cells, worse prognosis
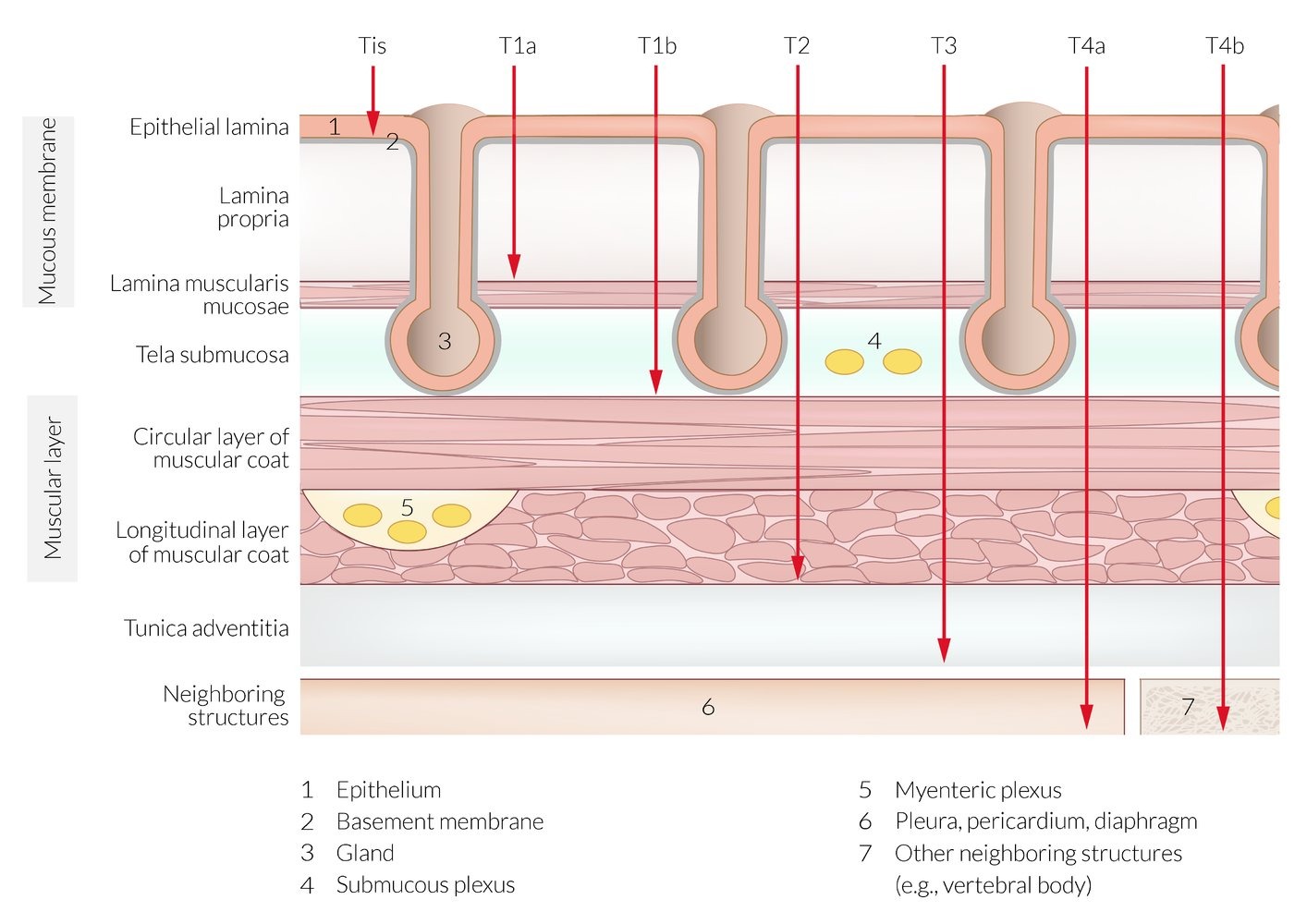
TYPES/CLASSIFICATION - was not included in the first version
| Gastric Cancer Classification | |||||
| Type | Frequency | Location | Key Features | Associated Conditions | Prognosis |
| Intestinal ★ | 54% | Lesser curvature, antrum | • Bulky, exophytic • Glandular structures • Well-differentiated |
• H. pylori • Chronic atrophic gastritis • Intestinal metaplasia |
Better (5-year survival: 20-25%) |
| Diffuse | 32% | Body, entire stomach | • Infiltrative growth • Signet ring cells • Linitis plastica |
• CDH1 mutation • Hereditary diffuse gastric cancer |
Worse (5-year survival: 10-15%) |
| Mixed | 14% | Variable | Features of both types | Variable | Intermediate |
Risk factor
- Gastric cancer is more common in Japan and Eastern Europe
- Helicobacter pylori (results in chronic gastritis secondary to increased production of proinflammatory proteins)
- Epstein-Barr virus (a rare cause of gastric adenocarcinoma)
- Nitrosamine exposure
- High salt intake
- Smoking tobacco
- Excessive alcohol use
Version 2
⚠️ High-Yield Risk Factors
- ★ Most common infectious cause: Helicobacter pylori (6-fold increased risk)
- ★ Most common premalignant lesion: Intestinal metaplasia
- ★ Most common genetic syndrome: Hereditary diffuse gastric cancer (CDH1 mutation)
Major Risk Factors:
- Infectious:
- H. pylori (★ most important modifiable risk factor)
- Epstein-Barr virus (10% of gastric cancers)
- Dietary:
- High salt intake
- Smoked/preserved foods (nitrosamines)
- Low fruit and vegetable intake
- Environmental:
- Tobacco smoking (1.5-fold increased risk)
- Excessive alcohol use
- Medical conditions:
- Chronic atrophic gastritis
- Pernicious anemia
- Prior gastric surgery (15-20 years post-op)
- Gastric polyps (adenomatous)
- Genetic:
- Family history (3-fold increased risk)
- Lynch syndrome
- Li-Fraumeni syndrome
Clinical features
- Symptoms
- Early satiety
- Leser-Trelat signal sudden development of seborrheic keratoses
- Acanthosis nigricans
- Dysphagia (gastric adenocarcinoma arise proximal)
- Persistent abdominal pain (commonly epigastric)
- Physical examination
- Unintentional weight loss (secondary to reduced caloric intake)
Version 2
🎯 Classic Presentation
"70-year-old man with early satiety, unintentional weight loss, and epigastric pain"
★ Most common symptoms (in order):
- Weight loss (60-70%)
- Abdominal pain (50-60%) - typically epigastric
- Early satiety (40-50%)
- Nausea/vomiting (30-40%)
- Dysphagia (25%) - if proximal/GE junction involved
Physical Examination Findings:
- ★ Most common finding: Epigastric tenderness
- Advanced disease signs:
- Virchow node (left supraclavicular lymphadenopathy)
- Sister Mary Joseph nodule (periumbilical nodule)
- Blumer shelf (palpable mass on rectal exam)
- Krukenberg tumor (bilateral ovarian metastases)
Paraneoplastic Syndromes:
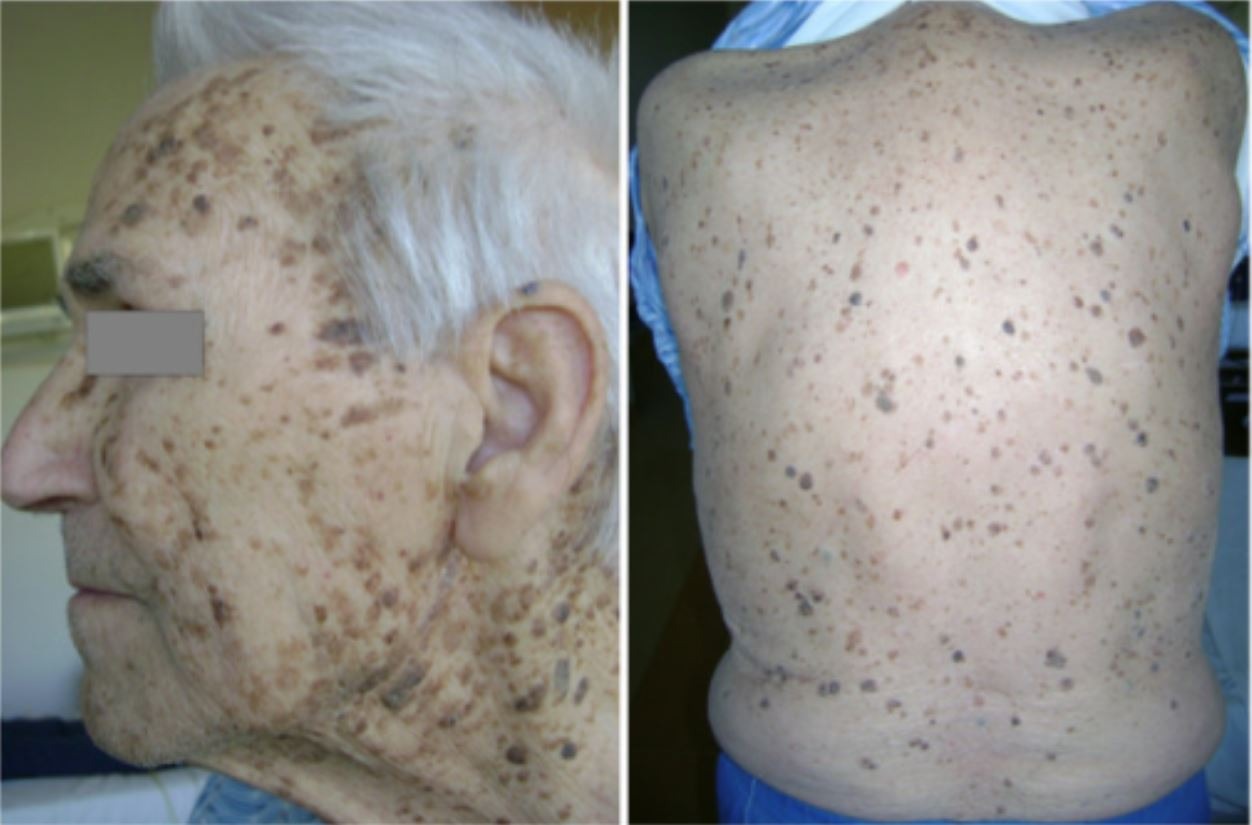
- Leser-Trélat sign: Sudden eruption of multiple seborrheic keratoses
- Acanthosis nigricans: Hyperpigmented, velvety skin changes
- Trousseau syndrome: Migratory thrombophlebitis
Diagnosis
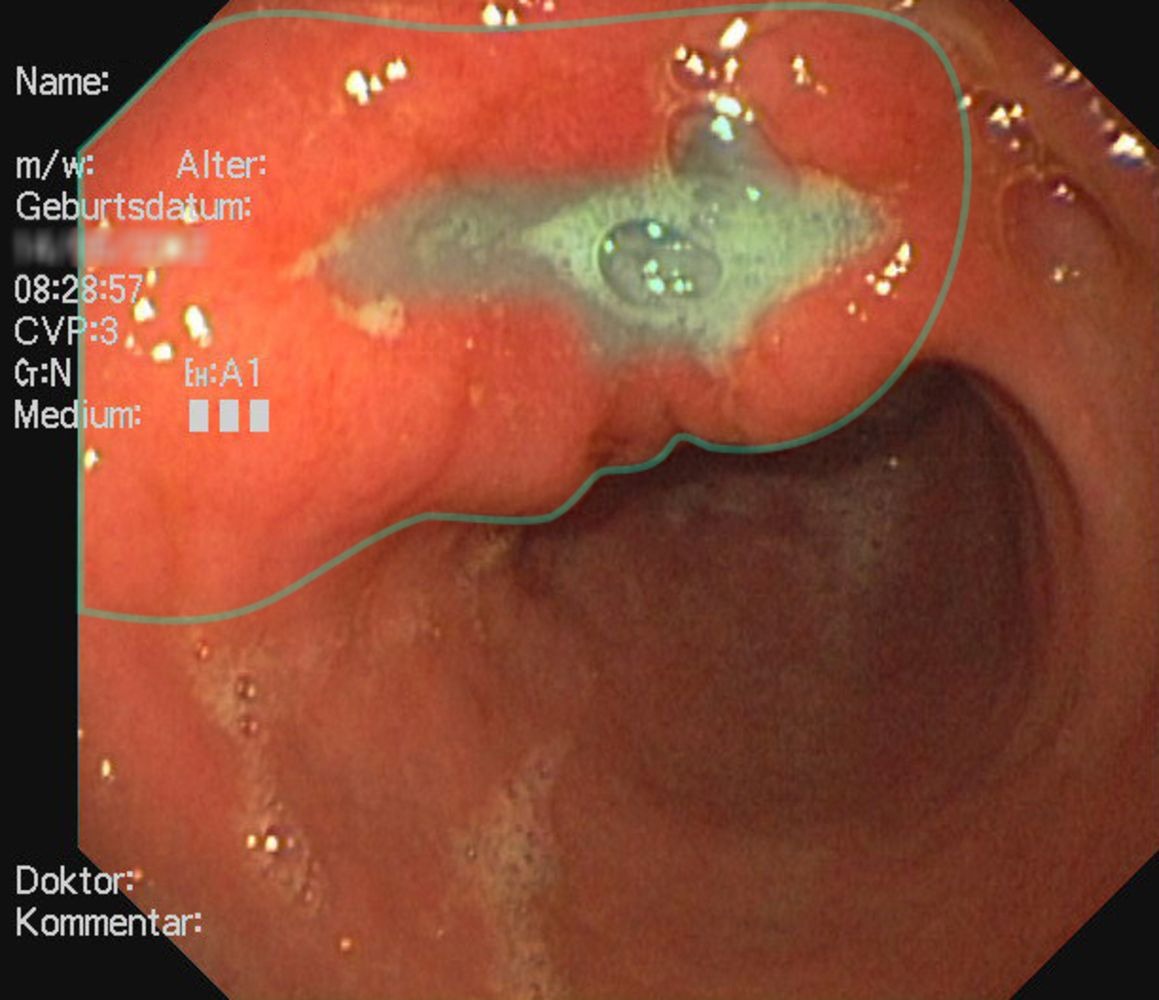
- Endoscopy and biopsy (findings include signet ring cells)
- Barium studies (it may be superior to endoscopy in detecting linitis plastica)
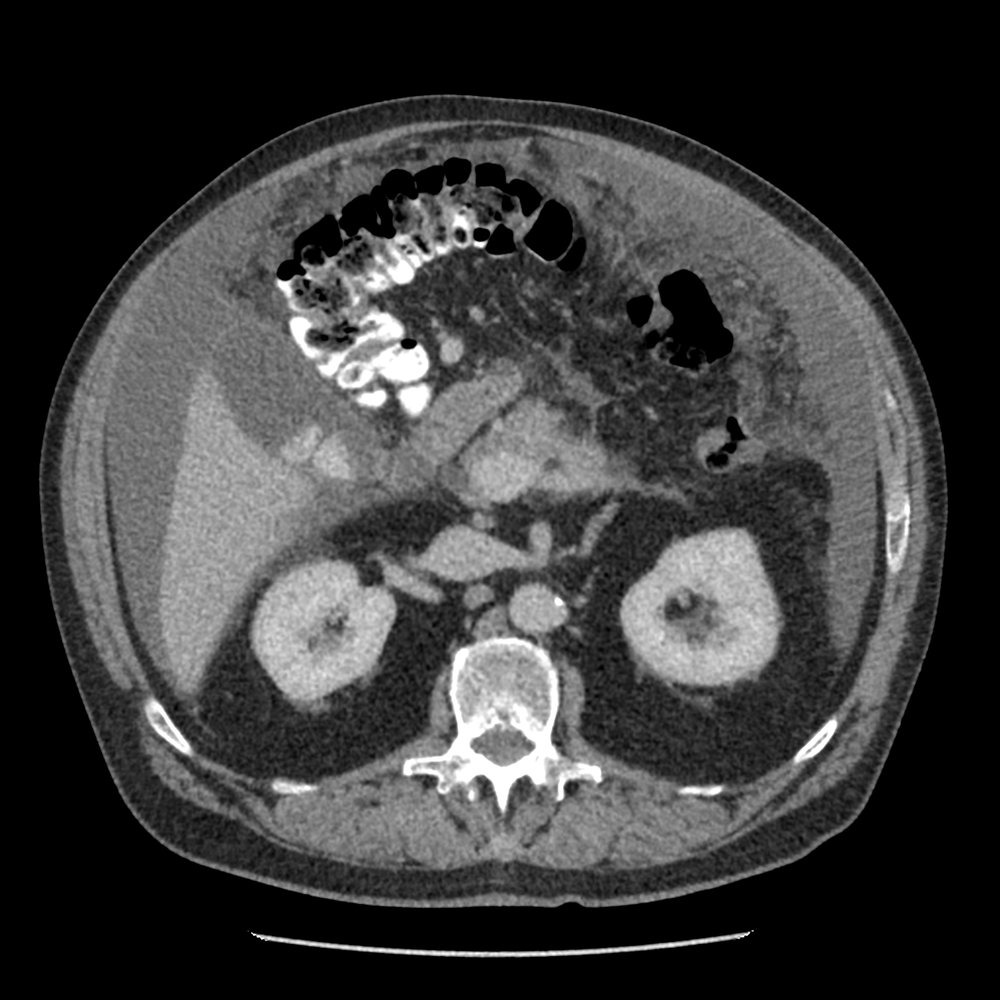
- PET scan and CT scan for staging
Version 2
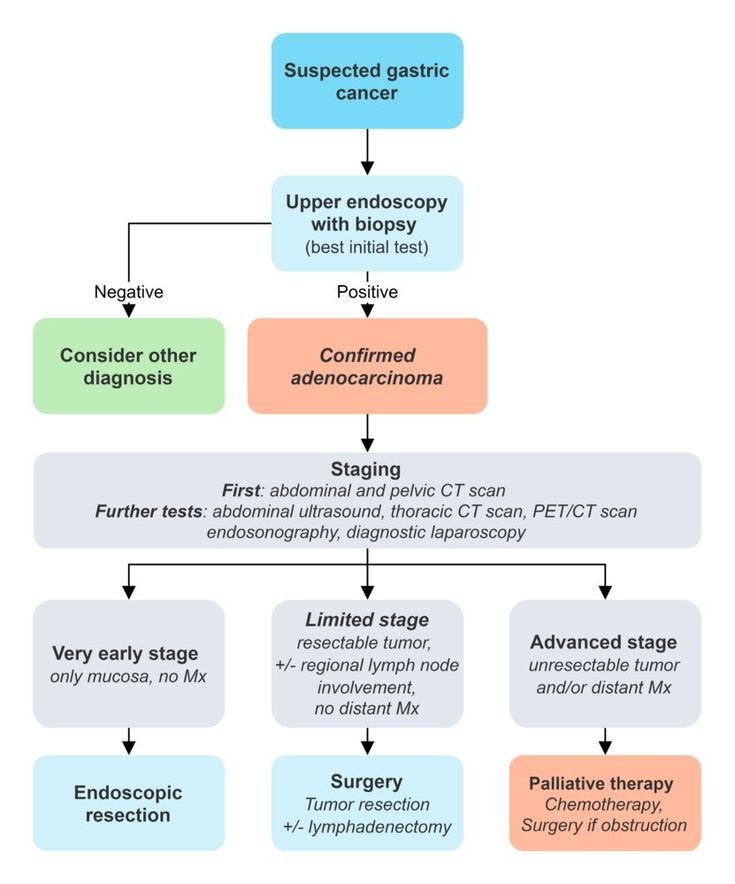
📋 Diagnostic Approach
- Best initial test: Upper endoscopy with biopsy
- Most accurate test: Histopathologic examination
- Best staging test: CT chest/abdomen/pelvis with contrast
Diagnostic Workup:
- Upper endoscopy with biopsy (★ gold standard)
- Multiple biopsies (minimum 6-8)
- Target suspicious areas and normal-appearing mucosa
- Histopathology findings:
- Intestinal type: Glandular structures
- Diffuse type: Signet ring cells (>50% of tumor cells)
- Staging studies:
- CT chest/abdomen/pelvis (initial staging)
- EUS (T and N staging)
- PET/CT (detect occult metastases)
- Diagnostic laparoscopy (detect peritoneal carcinomatosis)
Laboratory Studies:
- CBC (iron deficiency anemia in 40%)
- CEA, CA 19-9 (prognostic, not diagnostic)
- H. pylori testing
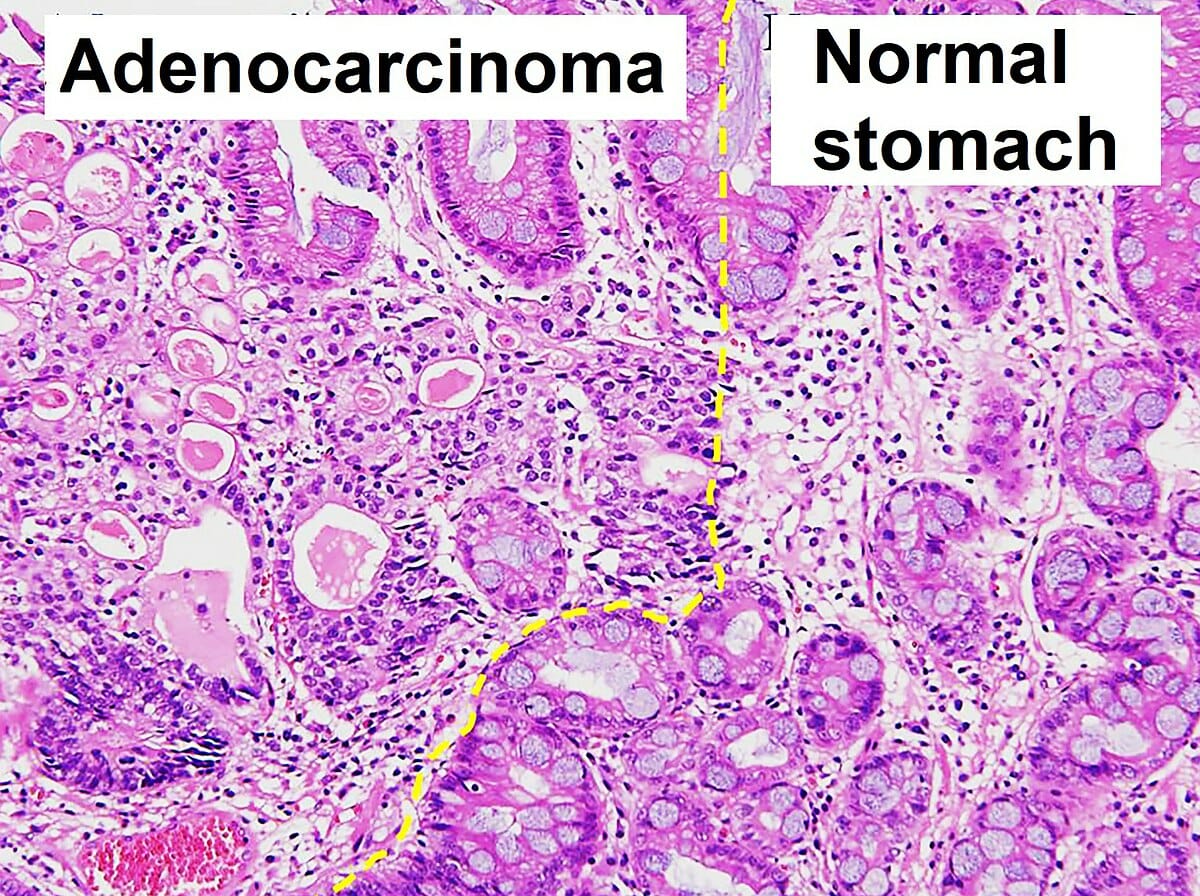
Differential diagnosis
- Gastric lymphoma (associated with mucosa-associated lymphoid tissue)
- Gastric stromal cancer
- Neuroendocrine (carcinoid) tumor
| Features of Carcinoid Syndrome | |
| Clinical manifestations |
|
| Diagnosis |
|
| Treatment |
|
Version 2
| Differential Diagnosis of Gastric Mass | |||
| Condition | Key Distinguishing Features | Diagnostic Test | Classic Association |
| Gastric lymphoma (MALT) | • Preserved mucosal folds • H. pylori association • May regress with antibiotics |
• Endoscopic biopsy • Immunohistochemistry |
H. pylori in 90% |
| GIST | • Submucosal mass • Smooth contour • c-KIT positive |
• EUS-guided FNA • CD117 staining |
Spindle cells |
| Peptic ulcer | • Regular borders • Clean base • Responds to PPI |
• Endoscopy • H. pylori testing |
NSAID use |
Treatment
- Tumor confined to mucosa (endoscopic resection)
- Gastrectomy with lymphadenectomy (for more extensive disease)
- Metastatic (palliative chemotherapy and radiation, surgery performed for cases of obstruction)
Version 2
💊 Treatment Algorithm
- Early stage (T1a): Endoscopic resection
- Localized (T1b-T4a): Surgery ± perioperative chemotherapy
- Metastatic: Palliative chemotherapy
Treatment by Stage:
-
Early gastric cancer (T1a, well-differentiated, <2cm):
- First-line: Endoscopic mucosal resection (EMR) or ESD
- Criteria: No lymphovascular invasion, <2cm, well-differentiated
-
Localized disease (T1b-T4a, N0-N3, M0):
- First-line: Gastrectomy with D2 lymphadenectomy
- Perioperative chemotherapy: FLOT regimen (5-FU, leucovorin, oxaliplatin, docetaxel)
- Alternative: MAGIC regimen (epirubicin, cisplatin, 5-FU)
-
Metastatic disease (M1):
- First-line chemotherapy:
- HER2-negative: FOLFOX or CAPOX
- HER2-positive: Trastuzumab + chemotherapy
- Second-line: Ramucirumab ± paclitaxel
- Third-line: Immunotherapy (pembrolizumab if MSI-H)
- First-line chemotherapy:
Complications
- Virchow note (left supraclavicular node involvement is secondary to metastasis)
- Krukenberg tumor (metastasis to both ovaries)
- Sister Mary Joseph nodule (periumbilical metastasis)
- Dumping syndrome (gastrectomy complications)
| Dumping syndrome | |
| Symptoms |
|
| Timing |
|
| Pathogenesis |
|
| Diagnosis |
|
| Initial management |
|
| Refractory cases |
|
Version 2
★ Most common complications:
- Gastric outlet obstruction (25-35%)
- Bleeding (20-30%)
- Perforation (1-2%)
Metastatic patterns:
- ★ Most common site: Liver (48%)
- Peritoneal carcinomatosis (35%)
- Lung (15%)
- Bone (10%)
Special metastatic sites:
- Virchow node: Left supraclavicular (via thoracic duct)
- Krukenberg tumor: Bilateral ovarian metastases (signet ring cells)
- Sister Mary Joseph nodule: Periumbilical metastasis
DUMPING SYNDROME
BACKGROUND
Definition: Dumping syndrome is a constellation of gastrointestinal and vasomotor symptoms resulting from rapid gastric emptying of hyperosmolar contents into the small intestine.
Epidemiology:
- ★ Most common cause: Post-gastric surgery (10-40% incidence)
- ★ Most common surgery associated: Roux-en-Y gastric bypass
- Onset: Can occur immediately post-op or years later
TYPES/CLASSIFICATION
| Types of Dumping Syndrome | ||||
| Type | Timing | Pathophysiology | Key Symptoms | Management |
| Early Dumping ★ | 10-30 minutes postprandial | • Hyperosmolar load • Fluid shift to intestine • GI hormone release |
• Abdominal pain • Diarrhea • Vasomotor symptoms |
• Small meals • Avoid simple sugars |
| Late Dumping | 2-3 hours postprandial | • Reactive hypoglycemia • Excessive insulin release |
• Hypoglycemic symptoms • Diaphoresis • Confusion |
• Complex carbohydrates • Acarbose |
CLINICAL FEATURES
🎯 Classic Presentation
"45-year-old woman 6 months post-Roux-en-Y gastric bypass with postprandial abdominal cramping, diarrhea, and palpitations occurring 20 minutes after meals"
Early Dumping Symptoms:
- GI symptoms (★ most common):
- Abdominal cramping
- Nausea
- Explosive diarrhea
- Bloating
- Vasomotor symptoms:
- Palpitations
- Diaphoresis
- Flushing
- Dizziness
- Syncope
Late Dumping Symptoms:
- Diaphoresis
- Tremor
- Confusion
- Hunger
- Weakness
- Syncope
DIAGNOSIS
📋 Diagnostic Criteria
- Best initial approach: Clinical diagnosis based on symptoms + surgical history
- Most specific test: Oral glucose tolerance test with HR monitoring
- Confirmatory test: Gastric emptying study
Diagnostic Tests:
-
Clinical diagnosis (usually sufficient)
- Characteristic symptoms
- Temporal relationship to meals
- History of gastric surgery
-
Oral glucose tolerance test:
- 50g glucose load
- Positive if: HR increase ≥10 bpm within 60 minutes (early dumping)
- Late dumping: Hypoglycemia at 2-3 hours
-
Gastric emptying study:
- Shows accelerated emptying
-
70% emptied at 30 minutes (normal <40%)
TREATMENT
First-line Management (Dietary Modifications):
- Small, frequent meals (6 small meals/day)
- Avoid simple sugars and high-osmolar foods
- Separate liquids from solids (drink 30-60 min after meals)
- Increase protein and fat intake
- Add soluble fiber (slows gastric emptying)
- Lie down after meals (delays gastric emptying)
Second-line (Pharmacologic):
- Octreotide (50-100 μg SC before meals)
- Mechanism: Delays gastric emptying, inhibits insulin release
- Reserved for refractory cases
- Acarbose (25-100 mg with meals)
- For late dumping/reactive hypoglycemia
- Delays carbohydrate absorption
Third-line (Surgical):
- Rarely needed (<1% of cases)
- Options: Pyloric reconstruction, conversion to Roux-en-Y
- Reserved for severe, refractory symptoms
⚠️ Clinical Pearl
Most patients (>85%) respond to dietary modifications alone. Symptoms typically improve over time as the GI tract adapts.
CARCINOID SYNDROME
BACKGROUND
Definition: Carcinoid syndrome is a paraneoplastic syndrome caused by systemic release of vasoactive substances (primarily serotonin) from well-differentiated neuroendocrine tumors (NETs).
Epidemiology:
- Occurs in 10% of patients with NETs
- ★ Most common primary site: Small intestine (45%), specifically ileum
- ★ Most common age: 50-60 years
- Prerequisite: Usually requires liver metastases (bypasses hepatic metabolism)
Pathophysiology:
- NETs produce serotonin, histamine, kallikrein, prostaglandins
- Normally metabolized by liver (first-pass effect)
- Liver metastases → systemic circulation → syndrome
- Exception: Bronchial NETs (direct systemic drainage)
CLINICAL FEATURES
🎯 Classic Presentation
"55-year-old man with episodic facial flushing, watery diarrhea, and wheezing, with symptoms triggered by alcohol or stress"
★ Classic Triad:
- Flushing (85-90%)
- Face, neck, upper chest
- Lasts 2-5 minutes
- Triggered by: alcohol, stress, exercise, certain foods
- Secretory diarrhea (70-80%)
- Watery, non-bloody
- 10-20 bowel movements/day
- Associated with cramping
- Bronchospasm/wheezing (15-20%)
Additional Features:
- Carcinoid heart disease (50% with syndrome)
- ★ Most common valve affected: Tricuspid (regurgitation > stenosis)
- Right-sided valvular fibrosis
- Plaque-like deposits
- Pellagra (5%) - niacin deficiency (4 D's: Dermatitis, Diarrhea, Dementia, Death)
- Mesenteric fibrosis → bowel obstruction
- Telangiectasias (chronic vasodilation)
DIAGNOSIS
📋 Diagnostic Approach
- Best initial test: 24-hour urine 5-HIAA
- Most sensitive imaging: Ga-68 DOTATATE PET/CT
- Cardiac screening: Echocardiogram (if 5-HIAA >300 μmol/24h)
Laboratory Tests:
- 24-hour urine 5-HIAA (★ gold standard)
- Sensitivity: 90%, Specificity: 90%
-
25 mg/24h (normal <10 mg/24h)
- Avoid serotonin-rich foods 3 days prior
- Plasma chromogranin A
- Elevated in 80-90% of NETs
- Non-specific (also elevated in PPI use, renal failure)
- Plasma serotonin (less reliable than 5-HIAA)
Imaging:
- CT/MRI abdomen - initial imaging
- Ga-68 DOTATATE PET/CT - most sensitive for NETs
- Echocardiogram - screen for carcinoid heart disease
Histopathology:
- Well-differentiated tumor cells
- "Salt-and-pepper" chromatin
- Positive for chromogranin A, synaptophysin
- Ki-67 index for grading
DIFFERENTIAL DIAGNOSIS
| Differential Diagnosis of Flushing + Diarrhea | |||
| Condition | Key Features | Distinguishing Test | Classic Trigger |
| Carcinoid syndrome | • Episodic flushing • Secretory diarrhea • Right heart disease |
↑ 24-hr urine 5-HIAA | Alcohol, stress |
| VIPoma | • Watery diarrhea • Hypokalemia • Achlorhydria |
↑ Plasma VIP | Fasting |
| Mastocytosis | • Urticaria • Hypotension • Bone pain |
↑ Serum tryptase | Physical stimuli |
| Pheochromocytoma | • Hypertension • Headache • Palpitations |
↑ Plasma metanephrines | Physical stress |
TREATMENT
💊 Treatment Goals
- Control symptoms
- Prevent carcinoid crisis
- Treat tumor (if possible)
- Manage complications
Symptomatic Management:
-
First-line: Somatostatin analogues
- Octreotide 100-500 μg SC TID or
- Lanreotide 90-120 mg deep SC q4 weeks
- Controls symptoms in 70-80%
- May slow tumor growth
-
Refractory diarrhea:
- Add telotristat 250 mg TID (tryptophan hydroxylase inhibitor)
- Loperamide, diphenoxylate
-
Flushing:
- Avoid triggers
- H1/H2 blockers for histamine-secreting tumors
Tumor-Directed Therapy:
- Localized disease: Surgical resection (curative intent)
- Metastatic disease:
- Peptide receptor radionuclide therapy (PRRT) with Lu-177 DOTATATE
- Hepatic artery embolization for liver metastases
- Everolimus (mTOR inhibitor)
- Cytotoxic chemotherapy (poorly differentiated only)
Carcinoid Crisis Prevention:
- High-dose octreotide (500 μg/hr IV) perioperatively
- Avoid triggers: catecholamines, histamine-releasing drugs
- Have vasopressors ready (avoid epinephrine)
COMPLICATIONS
★ Most common complication: Carcinoid heart disease (50%)
- Tricuspid regurgitation/stenosis
- Pulmonary valve involvement (less common)
- Right heart failure
- Treatment: Valve replacement when symptomatic
★ Most life-threatening: Carcinoid crisis
- Profound flushing, bronchospasm, hypotension
- Triggered by anesthesia, surgery, tumor manipulation
- Treatment: High-dose IV octreotide
📚 Memory Aid - Carcinoid Syndrome "5 S's"
- Serotonin secretion
- Secretory diarrhea
- Skin flushing
- Stenosis of right heart valves
- Somatostatin analogues for treatment
HIGH-YIELD EXAM FACTS
🎯 Must-Know Facts for Exams
- Gastric Cancer: - Most common type: Adenocarcinoma (90%) - Most common risk factor: H. pylori - Virchow node = left supraclavicular LN - Krukenberg tumor = bilateral ovarian mets with signet ring cells
- Dumping Syndrome: - Early (10-30 min) vs Late (2-3 hrs) - Diagnosis: Clinical (surgery + symptoms) - Treatment: Dietary modification first
- Carcinoid Syndrome: - Requires liver mets (except bronchial) - Diagnosis: 24-hr urine 5-HIAA - Right heart valves affected - Treatment: Octreotide
احصل على التجربة الكاملة
اشترك للوصول لفيديوهات الشرح التفصيلي والبطاقات التعليمية التفاعلية وأسئلة الممارسة مع تتبع التقدم.