YourMedPass
أكبر مرجع لامتحان الامتياز الأردني وامتحانات الإقامة
Celiac disease
Celiac disease, also referred to as celiac sprue or gluten-sensitive enteropathy, is a common chronic autoimmune disorder characterized by a maladaptive immune response to gluten, a protein found in wheat, barley, and rye. The disease frequently occurs in patients with other autoimmune conditions, as both are associated with HLA-DQ2 and HLA-DQ8 variants that cause pathologically increased immune responses to environmental antigens. The underlying pathophysiology involves gluten peptide (gliadin) deamidation by tissue transglutaminase, which triggers an autoimmune reaction and production of anti-tissue transglutaminase antibodies that target the proximal small intestine. This immune-mediated inflammation results in characteristic villous atrophy and crypt hyperplasia, leading to malabsorption syndrome with symptoms including chronic diarrhea, iron-deficiency anemia, weight loss, and extraintestinal manifestations such as dermatitis herpetiformis and neurologic symptoms. Diagnosis relies on serologic testing (anti-tissue transglutaminase IgA) followed by confirmatory small bowel biopsy showing characteristic histopathologic changes. A definitive diagnosis is essential, as treatment requires a strict lifelong gluten-free diet. With dietary compliance, the prognosis is excellent and the increased risk of celiac-associated malignancies (particularly enteropathy-associated T-cell lymphoma) is effectively mitigated.
Last updated: July 29, 2025
- Celiac disease (gluten-sensitive enteropathy, or celiac sprue) is a common chronic malabsorption syndrome that occurs due to an immune reaction to dietary gluten in genetically susceptible individuals. Chronic inflammation damages the small intestine and ultimately leads to symptoms of malabsorption.
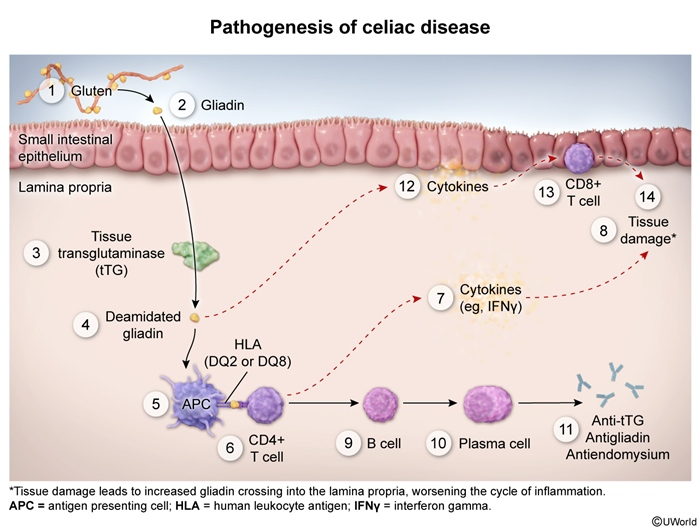
Sequential immune cascade triggered by gluten exposure:
- Gluten ingestion → Digestion to gliadin peptides
- Tissue transglutaminase deamidation of gliadin in small intestine
- HLA-DQ2/DQ8 presentation to CD4+ T cells
- Inflammatory cascade:
- Interferon-γ release → epithelial damage
- B cell activation → antibody production (anti-tTG, anti-endomysial)
- IL-15 release → CD8+ T cell recruitment
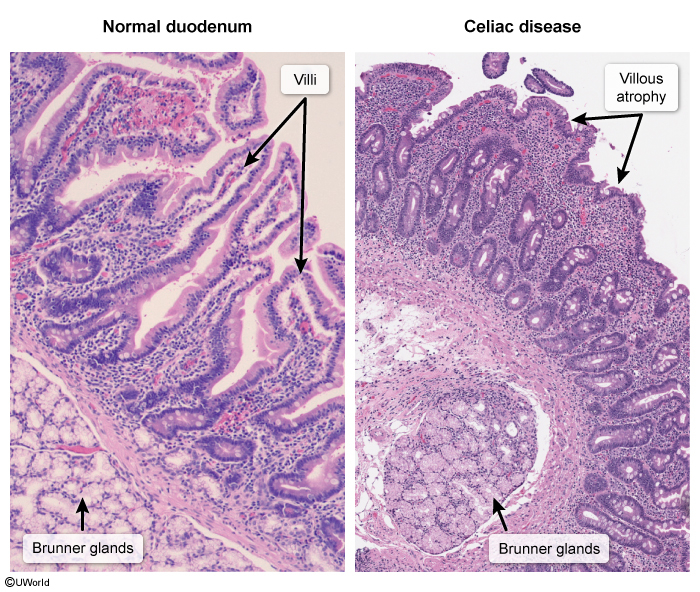
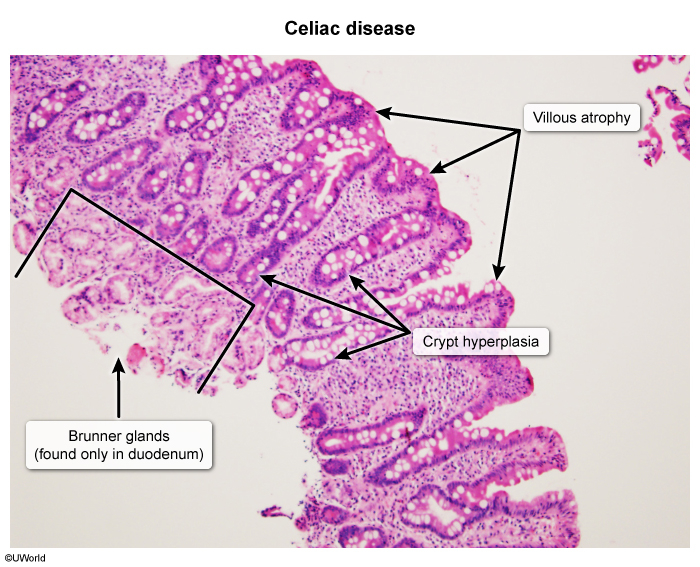
- Histologic changes: Villous atrophy + Crypt hyperplasia + Intraepithelial lymphocytosis ★
- Malabsorption syndrome (primarily duodenum/proximal jejunum)
In children, celiac disease presents after introduction of dietary gluten, usually between age 6 and 24 months. Infants and young children often develop classic symptoms of abdominal pain, bloating, osmotic diarrhea, and/or steatorrhea (due to malabsorption), and weight loss but may also present with failure to thrive (eg, declining linear growth velocity) or delayed puberty without associated gastrointestinal symptoms.
The clinical presentation of celiac disease in older children and adults may vary, including extraintestinal manifestations (eg, secondary to impaired micronutrient absorption) that can occur without overt gastrointestinal symptoms (). Extraintestinal manifestations may include:
- Micronutrient malabsorption (), particularly:
- Iron deficiency: Because iron is primarily absorbed in the duodenum, iron deficiency leading to microcytic anemia is a common manifestation of celiac disease; common presenting features include fatigue and pale mucosa.
- Vitamin D deficiency (): Vitamin D is a fat-soluble vitamin that is absorbed in the duodenum. Vitamin D deficiency results in calcium malabsorption, which interferes with bone mineralization, causing rickets (eg, leg bowing, costochondral hypertrophy) in children and osteomalacia (eg, bone pain, weakness) in adults. Calcium malabsorption also leads to secondary hyperparathyroidism. Parathyroid hormone induces bone resorption to mobilize calcium and phosphorus, which leads to osteopenia/osteoporosis and pathologic fractures. Parathyroid hormone also suppresses renal phosphate reabsorption. Laboratory evaluation reveals low serum 25-hydroxyvitamin D, high parathyroid hormone, normal or low calcium, and low phosphate.
- Dermatitis herpetiformis: characterized by erythematous pruritic papules, vesicles, and bullae that appear symmetrically on the extensor surfaces (eg, elbows, knees), upper back, and buttocks. The rash occurs due to cross-reactivity of tissue transglutaminase IgA antibodies with epidermal transglutaminase, resulting in microabscesses that contain fibrin and neutrophils at the dermal papillae tips (). The overlying basal cells become vacuolated, and coalescing blisters form at the tips of the involved papillae. Immunofluorescent imaging reveals deposition of IgA at the dermoepidermal junction.
- Peripheral neuropathy occurs in up to 50% of patients and may be due to autoantibody production, rather than nutritional deficiency, because peripheral neuropathy and other neuropsychiatric manifestations often precede symptoms of malabsorption.
Less commonly, patients can develop enamel hypoplasia and atrophic glossitis, possibly due to an autoimmune mechanism in addition to vitamin deficiencies.
Routine Studies
- IgA anti-tissue transglutaminase antibody (tTG-IgA) ★
- Initial test, widely available with high specificity (≥96%)
- Risk of false negatives (e.g., in IgA deficiency, gluten-free diet)
- Total IgA
- Must be ordered for all patients because of high prevalence of IgA deficiency in celiac disease (approx. 2-3%)
- If patients have low IgA, perform IgG-based testing (tTG-IgG, DGP-IgG)
Additional Studies
- Deamidated gliadin peptide (DGP): IgG-based testing for IgA deficiency or children <2 years
- HLA testing (HLA-DQ2/DQ8): Second-line testing for uncertain diagnosis or patients on gluten-free diet
- Anti-endomysial antibody (EMA): High-specificity confirmatory test
Endoscopy ★
- EGD with small intestine biopsy (confirmatory test)
Key Points
- Patient must consume ≥3g gluten daily for ≥6 weeks prior to testing ⚠️
- False-negative serology and histopathology possible if already on gluten-free diet
- Nutrient deficiency screening indicated after diagnosis (iron, vitamin D, B12, folate)
- Follow-up: Repeat tTG-IgA at 3-6 months, 12 months, then annually
- Education about the disease
- Life-long gluten-free diet
- Consultation with a dietitian
- Avoid barley, rye, and wheat.
- Many will have a secondary lactose intolerance
- Monitoring:
- Repeat IgA anti-tissue transglutaminase antibody at 6 and 12 months after diagnosis.
-
Small bowel biopsy after 3–6 months on a gluten-free diet
- Identify and treat any nutritional deficiencies (vitamin and mineral supplements, as needed).
- Most common reason for treatment failure is incomplete removal of gluten from the diet.
| Celiac disease | |
|---|---|
| Risk factors |
|
| Classic symptoms |
|
| Extraintestinal manifestations (may be sole presentation) |
|
| Diagnosis |
|
| Treatment |
|
| *Pediatric findings. | |