YourMedPass
أكبر مرجع لامتحان الامتياز الأردني وامتحانات الإقامة
Cushing syndrome
Cushing syndrome (hypercortisolism) reflects prolonged exposure to elevated systemic glucocorticoid levels. It can be categorized by whether it is due to excess adrenocorticotropic hormone (ACTH) secretion (ie, ACTH-dependent Cushing syndrome) or to autonomous, unregulated cortisol production (or exogenous glucocorticoids) (ie, ACTH-independent Cushing syndrome). Etiologies include:
- ACTH-dependent Cushing syndrome: ACTH-secreting pituitary adenoma (Cushing disease), ectopic ACTH production
- ACTH-independent Cushing syndrome: autonomous adrenal cortisol production (eg, adrenal adenoma, carcinoma), exogenous glucocorticoid administration
Hypercortisolism has widespread detrimental effects in multiple organ systems that can significantly impair quality of life and long-term health outcomes.
Last updated: July 20, 2025
- Cortisol is normally produced in the zona fasciculata of the adrenal cortex (). Production is regulated primarily by ACTH from corticotroph cells of the pituitary.
- Cushing syndrome results from excess cortisol production or exposure, which influences numerous metabolic, cardiovascular, immune, and musculoskeletal processes. Major effects of excess cortisol include the following:
- Metabolic: Stimulates gluconeogenesis, lipolysis, and proteolysis, causing hyperglycemia and central adiposity. Cross-stimulation of mineralocorticoid receptors by high cortisol levels causes sodium retention and potassium wasting.
- Cardiovascular: Major effects include hypertension, venous thromboembolism (possibly due to increased levels of clotting factors), and increased risk for coronary atherosclerosis.
- Immunosuppression: Inflammatory responses are suppressed, and susceptibility to infection increases.
- Musculoskeletal: Manifestations include muscle wasting and increased bone turnover with net bone loss (ie, osteoporosis).
- Cushing syndrome can be categorized with respect to ACTH as ACTH-independent or ACTH-dependent:
- ACTH-independent Cushing syndrome
- Autonomous cortisol secretion by adrenal tumors (adenomas, carcinomas) or adrenocortical hyperplasia suppresses pituitary ACTH secretion via negative feedback. This results in contralateral adrenal atrophy in unilateral adenomas.
- Exogenous Cushing syndrome (eg, due to chronic glucocorticoid therapy) is also associated with suppression of ACTH, as well as bilateral adrenocortical atrophy.
- ACTH-dependent Cushing syndrome
- ACTH stimulates bilateral adrenal hyperplasia and increased cortisol production. Major pathologic sources of ACTH are as follows:
- Pituitary adenoma (Cushing disease) is the most common cause of ACTH-dependent hypercortisolism, typically causing gradual symptom progression.
- Ectopic ACTH secretion arises from non-pituitary tumors (eg, small cell lung carcinoma, bronchial carcinoid). It is often associated with very high ACTH levels and a rapid onset of severe symptoms.
- ACTH stimulates bilateral adrenal hyperplasia and increased cortisol production. Major pathologic sources of ACTH are as follows:
- ACTH-independent Cushing syndrome
- ACTH is with melanocyte-stimulating hormone (MSH). Therefore, ACTH-dependent Cushing syndrome is often associated with hyperpigmentation, which may be most noticeable at the oral mucosa, palmar creases, surgical scars, sun-exposed areas, and nail beds. Hyperpigmentation is absent in ACTH-independent Cushing syndrome, in which ACTH secretion is suppressed. In addition, ACTH stimulates production of adrenal androgens (eg, dehydroepiandrosterone sulfate, androstenedione), which can lead to hirsutism and menstrual abnormalities in women.
| Clinical Pearl | |
|
ملاحظة |
Cushing syndrome manifests with widespread, multisystem symptoms and signs. Common findings include the following:
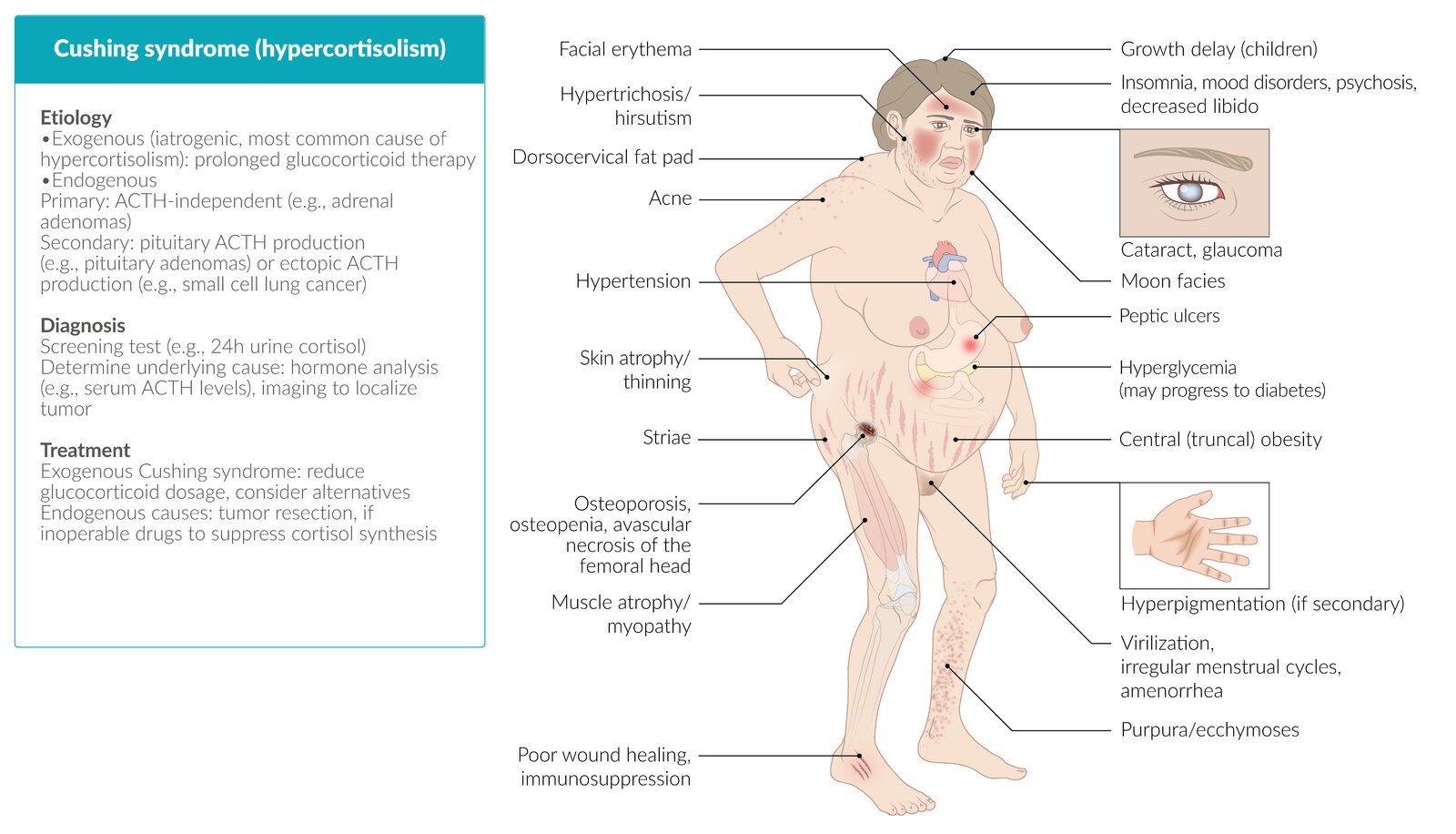
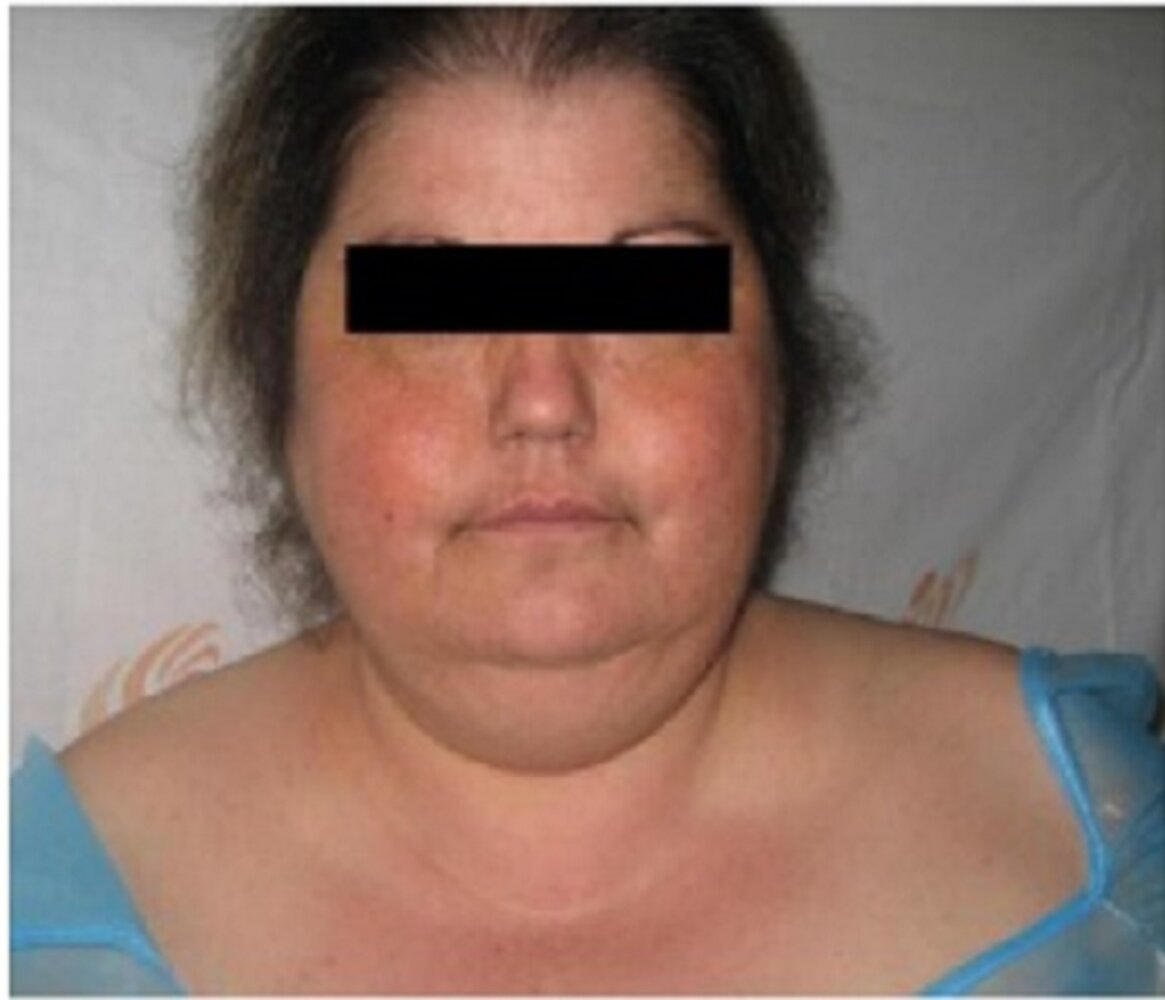
- Cushingoid body habitus: Central obesity, rounded "moon" facies, enlarged dorsocervical fat pad ("buffalo hump") ()
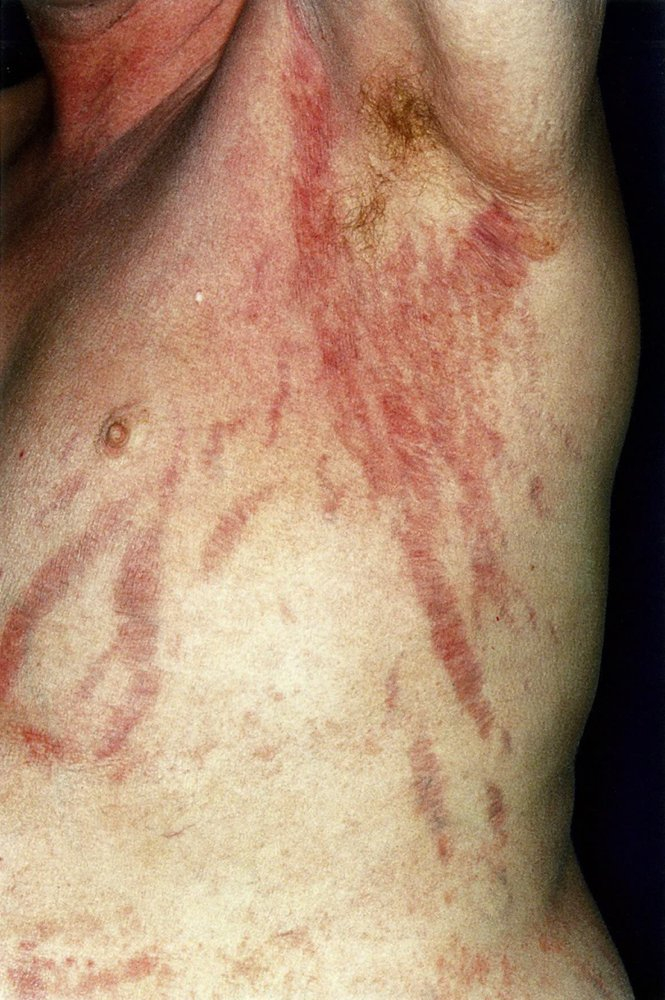
- Skin abnormalities: Wide (>1 cm) purple abdominal striae, thin and easily bruised skin, delayed wound healing, ecchymosis, hyperpigmentation (in ACTH-dependent cases), acanthosis nigricans, acne
- Cardiovascular features: (Secondary) hypertension
- Musculoskeletal features: Painless proximal muscle weakness and atrophy, osteoporosis
- Neuropsychiatric features: Anxiety, sleep disruption, mood changes, irritability, insomnia.
- Reproductive: Hyperandrogenism (eg, hirsutism, menstrual irregularities) in women, hypogonadism in men (due to suppression of pituitary gonadotropins)
- Metabolic abnormalities: Hyperglycemia, leukocytosis, possible hypernatremia and hypokalemia
Findings suggesting specific etiologies
- Ectopic ACTH secretion: Severe hypokalemia and hyperpigmentation. Rapid onset and severe clinical features are typical. Although central obesity is a common finding in Cushing syndrome of any cause, cachexia is often seen in patients with paraneoplastic Cushing syndrome due to the hypermetabolic effects of the underlying malignancy.
- Adrenal tumors: Unilateral mass on imaging and suppressed ACTH levels due to autonomous cortisol secretion. Hyperpigmentation and hypokalemia are generally absent.
The diagnosis of Cushing syndrome requires confirmation of excess cortisol production, determination of the etiology, and localization of the source (). As with most endocrine disorders, the metabolic evaluation should be performed prior to radiology studies because nonspecific imaging findings (eg, nonfunctioning adrenal adenomas) are common and can be misleading.
Serum cortisol levels have high variability and diurnal variation. Although a normal or elevated cortisol level is useful to rule out adrenal insufficiency, basal and spot cortisol levels are not recommended in evaluation of Cushing syndrome. Preferred options for initial testing include 24-hour urine free cortisol, late-night (ie, bedtime) salivary cortisol, and the low-dose dexamethasone suppression test. If suspicion is low, a single test may be adequate to rule out Cushing syndrome, with a second test performed if the first is positive. However, if suspicion is high, 2 tests should be performed initially. Testing options include the following:
- First-line (screening) tests:
- 24-hour urinary free cortisol: A 24-hour collection can account for variations in circulating cortisol levels through the day. Unequivocally elevated levels (eg, ≥3 times the upper limit of normal) confirm cortisol overproduction.
- Late-night salivary cortisol: Cortisol production is normally low late in the day, and elevated levels indicate loss of diurnal cortisol variation. Salivary samples generally parallel blood levels but can be collected easily by the patient at home without phlebotomy. This test is often performed on 2 samples to improve yield.
- Low-dose dexamethasone suppression test: Oral dexamethasone is administered, and serum cortisol measured the following morning. Dexamethasone is a synthetic glucocorticoid that suppresses normal endogenous cortisol secretion but does not cross-react on laboratory assays for cortisol. A failure to suppress serum cortisol suggests Cushing syndrome.
- ACTH measurement: For patients with confirmed hypercortisolism (ie, multiple positive first-line tests, sustained over a period of multiple weeks), plasma ACTH measurement is indicated.
- Elevated ACTH indicates ACTH-dependent causes (pituitary or ectopic ACTH secretion).
- Low ACTH suggests ACTH-independent causes (adrenal tumors or hyperplasia).
- High-dose dexamethasone suppression test in ACTH-dependent Cushing syndrome (): Follow-up testing is indicated to differentiate an ACTH-secreting pituitary adenoma (Cushing disease) from ectopic (paraneoplastic) ACTH secretion. In Cushing disease, ACTH and cortisol production can usually be suppressed by high-dose dexamethasone (eg, 8 mg), whereas ectopic ACTH production is usually completely nonsuppressible. Therefore, a high-dose dexamethasone suppression test can often differentiate the likely source of ACTH.
- Localization studies:
- Pituitary MRI: Preferred for identifying ACTH-secreting pituitary adenomas.
- Chest and abdominal CT: Used to identify ectopic ACTH-secreting tumors or adrenal masses.
- Inferior petrosal sinus sampling: Differentiating between pituitary and ectopic ACTH secretion when imaging is inconclusive.
General measures for all patients with Cushing syndrome include managing complications such as hyperglycemia, hypertension, and osteoporosis. Additional measures are determined by the underlying cause.
ACTH-dependent causes
- Cushing disease: Transsphenoidal resection of the pituitary adenoma is the treatment of choice.
- Ectopic ACTH secretion: Surgical removal of the ectopic tumor should be performed, if feasible. Medical therapy (eg, ketoconazole, metyrapone) may be required for inoperable cases.
ACTH-independent causes
- Adrenal tumors (adenomas, carcinomas): Surgical adrenalectomy is indicated.
- Bilateral adrenal hyperplasia: May require medical therapy to suppress adrenal cortisol production (eg, mifepristone, mitotane).
Iatrogenic Cushing syndrome
Cushing syndrome due to exogenous glucocorticoids is usually associated with significant adrenal suppression and atrophy, which does not recover immediately after cessation. Therefore, gradual tapering of glucocorticoids is needed to prevent acute adrenal insufficiency (adrenal crisis).
Cushing syndrome () is characterized by cortisol excess, manifesting with central obesity, hypertension, hyperglycemia, and distinctive skin and musculoskeletal findings. Besides exogenous glucocorticoid administration, the most common etiologies include increased pituitary secretion of ACTH (Cushing disease), cortisol-producing adrenal tumors, and ectopic ACTH production in a malignancy (eg, small cell lung cancer).
Diagnosis relies on biochemical confirmation of excessive cortisol production with 24-hour urinary cortisol, late-night salivary cortisol level, and/or low dose dexamethasone suppression test. If Cushing syndrome is confirmed, follow up testing includes ACTH measurement and imaging studies to identify the etiology. Management is etiology-specific and often requires surgical intervention. Prompt recognition and treatment improve outcomes, but long-term complications can include obesity, osteoporosis, hypertension, and diabetes mellitus.
| Mnemonic for Cushing Syndrome Features | |
|
CUSHING Mnemonic for Clinical Features
|
جملة تذكرية |
| Mnemonic for Diagnostic Tests | |
|
SALUD Mnemonic for Screening Tests
|
جملة تذكرية |
| Mnemonic for Ectopic ACTH Features | |
|
RAPID Mnemonic for Ectopic ACTH Syndrome
|
جملة تذكرية |
| Mnemonic for ACTH-Dependent vs Independent | |
|
PECS Mnemonic for ACTH Classification
|
جملة تذكرية |